While most stormwater samples get sent off to an accredited lab, pH is one of the few parameters that can be measured in the field. The process for analyzing pH onsite is relatively simple, however, there are a few considerations to keep in mind to help us ensure that our results are accurate and that sampling goes smoothly. When measuring pH, we want to make sure we are using the right equipment, that our equipment is properly calibrated, and that we measure our samples quickly after sampling.
1. Equipment Considerations
There are several equipment options for pH measurements in the field:
Handheld, portable pH meters offer a high degree of accuracy and precision but are the most expensive of the options considered here. Some of these have data-logging capabilities, which can make things like source evaluation and long-term monitoring a breeze. The steep price tag usually keeps these meters reserved for only the most rigorous testing needs.
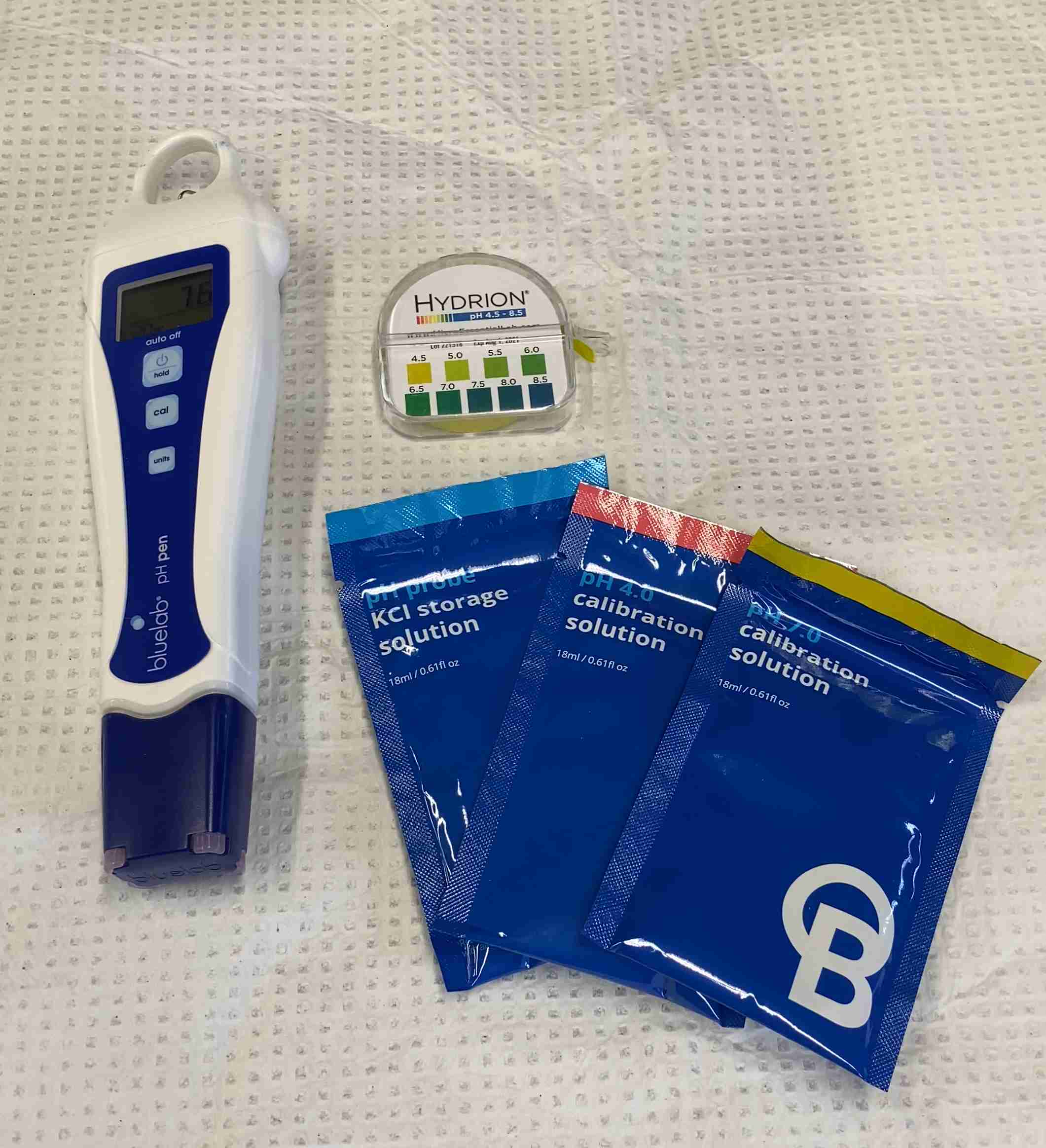
pH hand-held “pens” are commonly used by industrial permittees. While these pens don’t offer all of the features that pH meters offer, they are suitable for most purposes. These pens are simple to use and provide accurate results.
pH test strips are another common method of testing and are cheap and straightforward to use. pH strips are coated with a chemical indicator; when dipped in water, they change color depending on the pH. The resulting color is visually matched against a reference chart (provided by the manufacturer) to determine the pH value. For purposes of the ISGP, strips should be within a narrow range and give the precision of +/- 0.5 s.u.
2. Calibrating and storing equipment
pH meters and hand-held pens should be calibrated frequently. In most cases, the calibration procedure requires you to insert your meter into one or a series of calibration standards while running a calibration function within the device. Exact procedures will vary from instrument to instrument, so follow the manufacturer’s instructions for your specific device. Calibrations are relatively quick and simple to perform and ensure the quality of your measurements. Therefore, we recommend performing these calibrations before every sampling event. If you have a lot of samples, you may also consider periodically measuring the pH of the buffer solutions to check that your instrument’s calibration has not drifted. Re-calibrate as needed.

It is also important to check the quality of your calibration standards. These standards can come in sizes ranging from single-use sachets to large bottles. Only buy enough for what you can use in a year, as these standards have a limited shelf life. Check and make note of the expiration dates of these samples so you can have fresh supplies ordered in time for your next sampling event.

pH probes should also be stored in a storage solution. This solution is usually a mixture of KCl and water and keeps the probe moist and in the ionic conditions required to keep it functioning properly. These storage solutions have a little longer shelf life than the calibration standards but should be replaced as needed. It is a good idea to keep extra solutions on hand just in case you knock over the storage container.
4. Sampling for pH
When measuring pH in the field, make sure you have a clean sampling bottle or container dedicated to this measurement. This step is easily forgotten, as in most cases your lab provides you with bottles for your other parameters. It can be very easy to forget your bottle, and tempting to just dip your test probe or test strip into a bottle intended for another parameter. Doing so will risk cross-contamination of samples collected for other lab analyses. If using a pH meter or pen, further avoid cross-contamination by rinsing the probe between each sample.
Once you have collected your water sample in the bottle designated for pH, take the pH measurement as soon as possible following sample collection. pH will change over time as carbon dioxide from the air dissolves in the water, therefore the maximum amount of time between sample collection and pH testing should be 15 minutes.

5. Organization
It is easy to lose track of your pH measurements during the hustle and bustle of a sampling day. This is often the only parameter not measured by the lab and sent to you in a nicely organized lab report. Before going out into the field, you should have a dedicated place to record your results in the field and a procedure to organize those results and enter them into your Discharge Monitoring Report.
For more video content covering a wide range of stormwater topics, please visit our YouTube page!



